PD Functional Genomics | 2020
Mechanisms Overwhelming Protein and Organelle Quality Control in Parkinson’s Disease
Study Rationale: Abnormal protein aggregation and prion-like aggregate spreading are hallmarks of the degenerative cascades of sporadic and familial Parkinson’s disease (PD) and can damage cells, including neurons. Multiple mechanisms of aggregate toxicity have been implicated in cellular PD pathology, and PD risk alleles may have the potential to illuminate additional underlying biological mechanisms.
Hypothesis: Parkinson’s disease, at the molecular level, results from the failure of cellular quality control (QC) mechanisms, and finding ways to maintain (or augment) QC capacity will provide new therapeutic strategies for PD and possibly other neurodegenerative disorders.
Study Design: Using powerful molecular visualization and discovery tools in disease-relevant cells, Team Harper will elucidate how individual types of protein aggregates linked with PD strains (including patient-derived aggregates) alter cellular pathways, including effects on cell survival and function. Team Harper will also use genetic approaches to understand what cellular proteins promote the processing of PD-related aggregates.
Impact on Diagnosis/Treatment of Parkinson’s Disease: Team Harper’s expectation is that this work will identify those critical cellular functions that are disrupted by protein aggregates and will help define how mutations alter the underlying mechanisms of dysfunctional proteostasis.
Leadership
Project Outcomes
Through biochemical reconstitution, in situ structural analysis, and genetic perturbations, this project will directly visualize pathogenic mechanisms in cells and in reconstituted systems at nanometer and subnanometer resolution, providing an unprecedented understanding of how a-synuclein strains and other PD mutants promote cellular dysfunction. View Team Outcomes.
Team Outputs
Click the following icons to learn more about the team’s outputs:
Overall Contributions
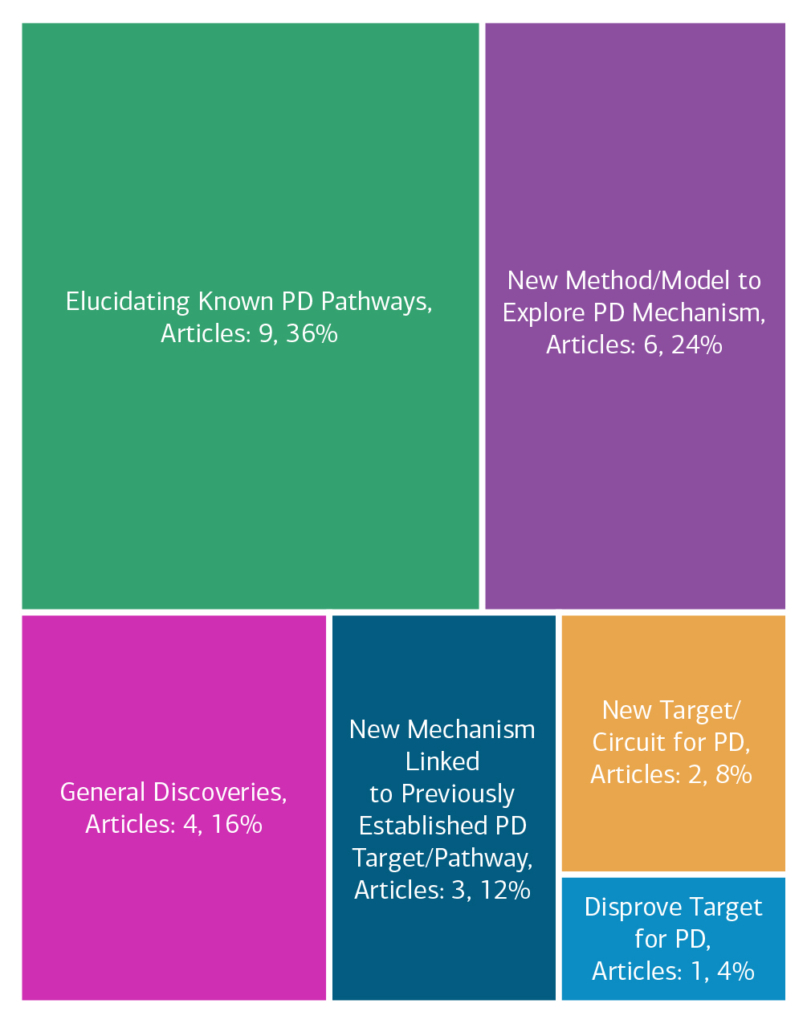
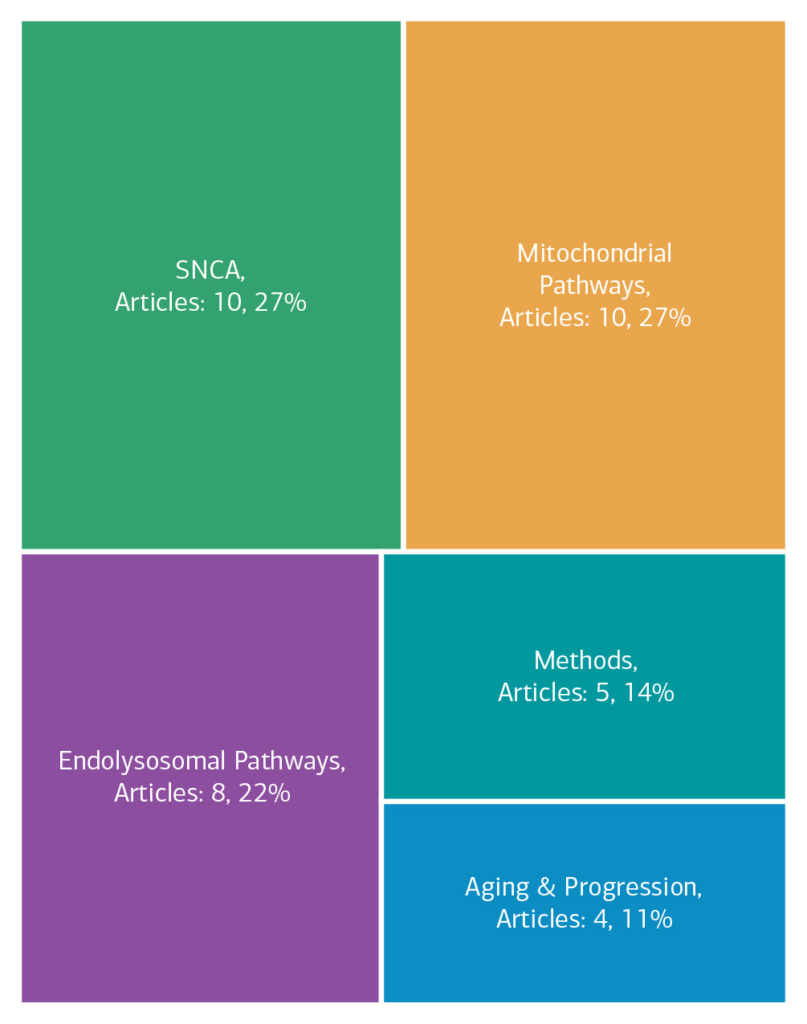
Here is an overview of how this team’s article findings have contributed to the PD field as of May 2025. There are two different categorizations of these contributions – one by impact to the PD community and a second by scientific category.
Impact
Category
Featured Output
Below is an example of a research output from the team that contributes to the ASAP mission of accelerating discoveries for PD.
α-Synuclein aggregates inhibit ESCRT-III through sequestration and collateral degradation
α-Synuclein aggregation is a hallmark of Parkinson’s disease and related synucleinopathies. Intracellular aggregates sequester numerous proteins, including subunits of the ESCRT-III system for endolysosome membrane repair, but the toxic effects of these events remain poorly understood.The authors found that α-synuclein fibrils interact with an α-helix common to ESCRT-III proteins, which results in sequestration of ESCRT-III subunits and triggers their proteasomal destruction in a process of “collateral degradation.” These twin mechanisms deplete the available ESCRT-III pool, initiating a toxic feedback loop. The ensuing loss of ESCRT function compromises endolysosome membranes, thereby facilitating escape of aggregate seeds into the cytoplasm, which in turn increases aggregation and ESCRT-III sequestration. They suggest that collateral degradation and triggering of self-perpetuating systems could be general mechanisms of sequestration-induced proteotoxicity.
Team Accolades
Members of the team have been recognized for their contributions.
- Open Science Champions: Felix Kraus, Harper Lab, Cristina Capitanio
- Network Spotlights: Felix Kraus
- Awards
- 2023 Collaborative Meeting: Cole Sitron (Community Poster Award Winner; London)

Other Team Activities
- Protocol Particulars Interview: iNeuron Differentiation on EM Grids with Cristina Capitanio, MSc
- Interest Groups:
- Mitochondrial Pathways – J. Wade Harper (Past Chair)
- Endolysosomal Pathways – J. Wade Harper (Chair)
In the News
- Check back soon for updates from Team Harper.