Neuro-immune Interactions | 2020
The Genome-Microbiome Axis in the Cause of Parkinson Disease: Mechanistic Insights and Therapeutic Implications from Experimental Models and a Genetically Stratified Patient Population
Study Rationale: Mutations in the gene for glucocerebrosidase (GBA) increase alpha-synuclein expression and are common in Parkinson disease (PD). However, only about a third of people with GBA mutations get PD. Research suggests that the increase in the alpha-synuclein protein associated with PD may come from the gut and travel along nerves that go to the brain. The microbiome environment, including an increase in gut alpha-synuclein and inflammation, may be a causal link between GBA mutations and PD.
Hypothesis: Team Schapira thinks that the combination of one’s genetic makeup and microbiome are important in their risk for getting PD. The team will look at people with GBA mutations to see if their risk for PD is caused by their gut bacteria, and to see if their bacteria increase alpha-synuclein transport from gut to brain.
Study Design: Team Schapira will use mouth and fecal samples from people with GBA-PD to identify the bacteria special to them and how these might increase alpha-synuclein and cause PD. The team will also use special lab models to study the changes that link the bacteria and inflammation to alpha-synuclein and its spread from the gut to the brain. Team Schapira will explore methods to change the bacterial composition of the microbiome to see if this can stop alpha-synuclein transport to the brain.
Impact on Diagnosis/Treatment of Parkinson’s Disease: This research may provide insight into which GBA mutations carriers are most likely to get PD and whether bacterial profile changes will alter alpha-synuclein transport. Being able to predict PD onset may allow Team Schapira to find a treatment window to prevent disease onset altogether. Further, this research may open more avenues for drug repurposing to find compounds that alter microbiome composition in ways that are beneficial for halting alpha-synuclein transport.
Leadership

Co-PI (Core Leadership)
Mathieu Almeida, PhD
INRAE (National Research Institute for Agriculture, Food and the Environment)
Project Outcomes
Team Schapira will clarify the role of intestinal bacteria in Parkinson disease, understand its interaction with glucocerebrosidase mutations – a genetic cause of the disease, and provide insight into potential new therapeutic targets. View Team Outcomes. View Team Outcomes.
Team Outputs
Click the following icons to learn more about the team’s outputs:
Overall Contributions
Here is an overview of how this team’s article findings have contributed to the PD field as of June 2025. There are two different categorizations of these contributions – one by impact to the PD community and a second by scientific category.
Impact
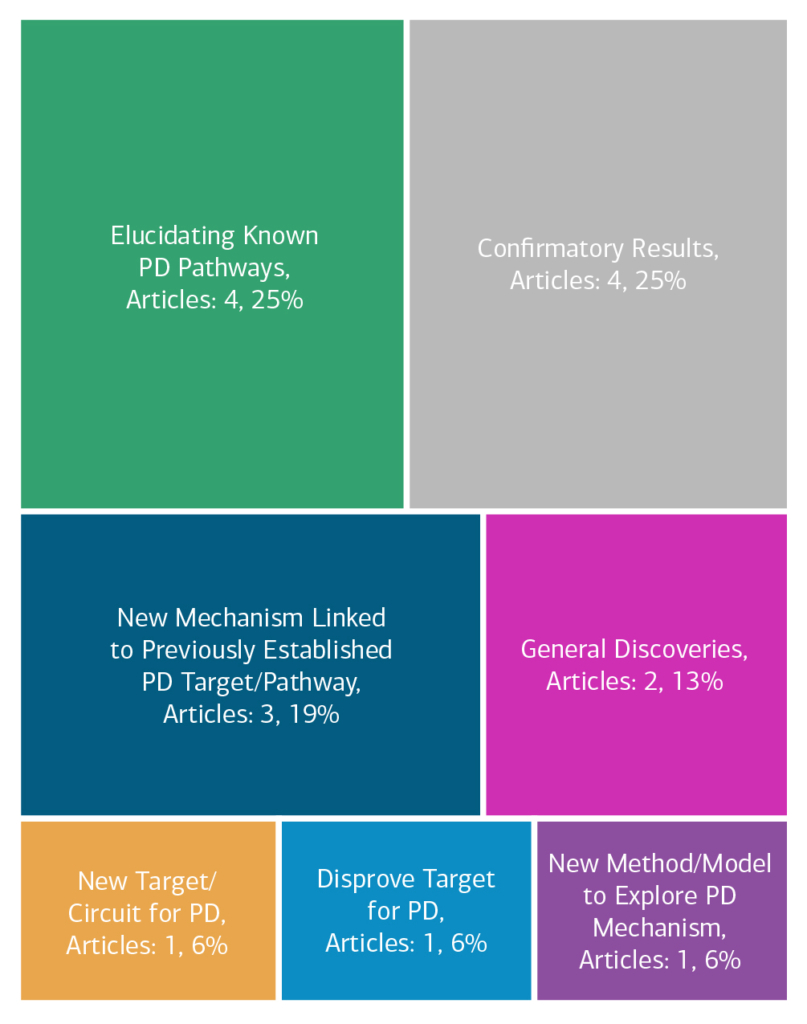
Category

Featured Output
Below is an example of a research output from the team that contributes to the ASAP mission of accelerating discoveries for PD.
Mitochondrial oxidant stress promotes α-synuclein aggregation and spreading in mice with mutated glucocerebrosidase
In this study, heterozygous expression of a common glucocerebrosidase variant, namely the L444P mutation, was found to exacerbate α-synuclein aggregation and spreading in a mouse model of Parkinson-like pathology targeting neurons of the medullary vagal system. These neurons are primary sites of α-synuclein lesions in Parkinson’s disease and were shown here to become more vulnerable to oxidative stress after L444P expression. Nitrative burden paralleled the enhanced formation of reactive oxygen species within vagal neurons expressing mutated glucocerebrosidase, as indicated by pronounced accumulation of nitrated α-synuclein. Scavenging of oxygen species by superoxide dismutase 2 effectively counteracted deleterious nitrative reactions and prevented nitrated α-synuclein burden. Together, these findings support the conclusion that enhanced vulnerability to mitochondrial oxidative stress conferred by glucocerebrosidase mutations should be considered an important mechanism predisposing to Parkinson’s disease pathology, particularly in brain regions targeted by α-synuclein aggregation and involved in α-synuclein spreading.
Team Accolades
Members of the team have been recognized for their contributions.
- Open Science Champions: Michael Hurley
- Network Spotlights: Michela Deleidi, Marco Toffoli, Sofia Koletsi, Mathieu Almeida, Anthony Schapira, Sara Lucas Del Pozo
- Awards
- 2023 Collaborative Meeting: Christin Weissleder (Third Place Winner in Neuro-Immune; London)
- COSA Prize Winners 2022: Hariam Raji (Second Place)
- COSA Prize Winners 2024: Maria Jose Perez Jimenez (Third Place)
Other Team Activities
- Protocol Particulars Interview: Isolation of PBMCs with Gerardo Ongari, PhD
- Working Groups:
- Clinical & Immune – Anthony Schapira (Co-Chair)
- Microbiome – Mathieu Almeida (Co-Chair)
- Interest Groups: Gut/Brain, Microbiome, and Clinical Biomarkers – Anthony Schapira (Co-Chair)
In the News
- University College London: Parkinson’s disease research at UCL gets £19M boost (University of College London, press release, September 16, 2020)