PD Functional Genomics | 2020
Parkinson5D: Deconstructing Proximal Disease Mechanisms Across Cells, Space, and Progression
Study Rationale: Genome-wide association studies (GWAS) have unequivocally linked thousands of noncoding variants in ninety independent GWAS signals to susceptibility for common, genetically complex Parkinson’s disease (PD) that affects more than 7 million people around the world. Why have these breakthroughs not uncovered the mechanism(s) of PD? Researchers do not know how disease-associated variants cause neurodegeneration and why they impair some brain cells but not others. Team Scherzer’s research will tackle the critical task of clarifying the precise mechanisms through which this wealth of genetic variation regulates onset and progression of PD.
Hypothesis: Team Scherzer hypothesizes that most GWAS variants function through cell-, space-, and stage-dependent gene-regulatory mechanisms.
Study Design: Here Team Scherzer will develop a molecular atlas of PD that reveals how GWAS/familial genetics control proximal disease mechanisms in five dimensions: brain cells (1D), brain space (3D), and disease stage (1D). The team will reveal how genetic variants modulate mechanisms in specific brain cells in specific topographic locations of midbrain and cortex during the progression of neuropathology from healthy brains to prodromal to symptomatic disease. Massively parallel analysis of hundreds of thousands of single human brain cells with genetic transcriptomics, high-resolution spatial transcriptomics, and fine-mapping of causal alleles with allelic imbalance in human brains will be combined with the prodigious power of cell- and stage-specific mechanistic analyses in brain of Drosophila avatars and in vitro in human pluripotent stem cells.
Impact on Diagnosis/Treatment of Parkinson’s Disease: Team Scherzer’s collaborative and integrative project will translate the complex human genetics of PD into a dynamic, five-dimensional view of proximal cellular mechanisms. It will begin to reveal how single nucleotide variation in a person’s universal DNA code regulates gene activity (without changing protein sequence) in situ in billions of physiologically specialized neurons and glia cells, and determines, how, when, which, and where brain cells are destined to malfunction.
Leadership
Project Outcomes
Parkinson5D will translate the complex human genetics of Parkinson’s disease into a dynamic, spatiotemporal understanding of proximal mechanisms in specific brain cells --- in situ in patients’ brains, in vivo in Drosophila, and in vitro using human pluripotent stem cell genetics. It will begin to reveal how single nucleotide variation in a person’s universal DNA code regulates gene activity in situ in billions of physiologically specialized neurons and glia cells, and determines, how, when, which, and where brain cells are destined to malfunction. View Team Outcomes.
Team Outputs
Click the following icons to learn more about the team’s outputs:
Overall Contributions
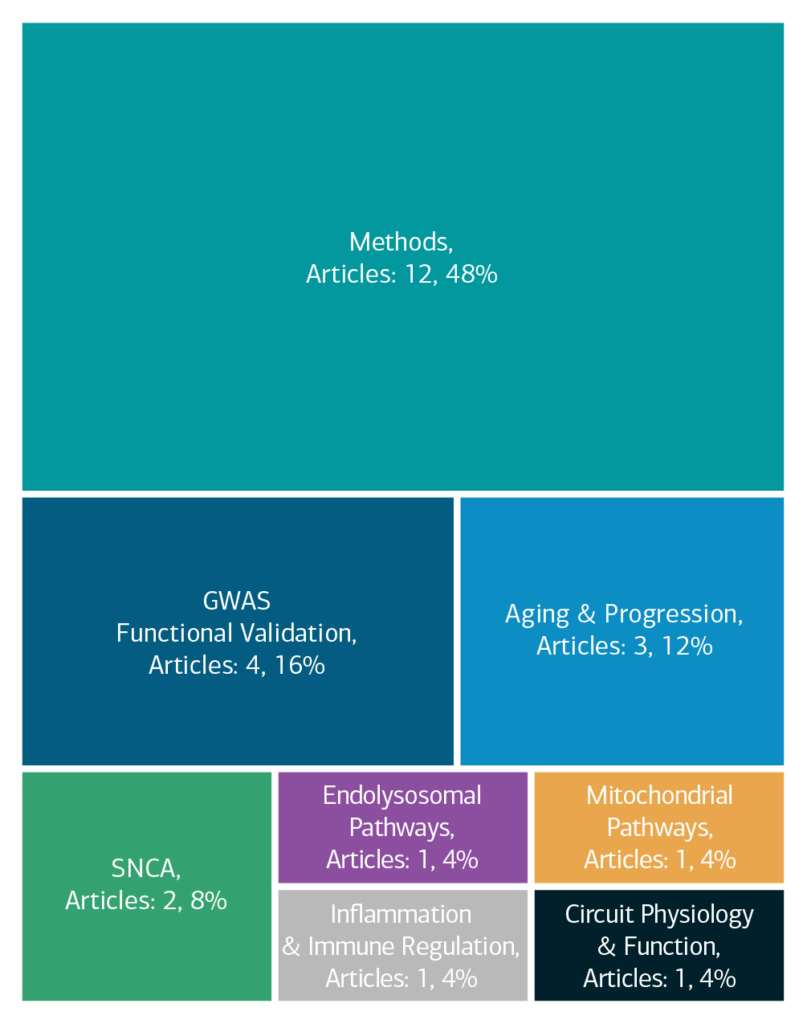
Here is an overview of how this team’s article findings have contributed to the PD field as of May 2025. There are two different categorizations of these contributions – one by impact to the PD community and a second by scientific category.
Impact

Category
Featured Output
Below is an example of a research output from the team that contributes to the ASAP mission of accelerating discoveries for PD.
Circular RNAs in the human brain are tailored to neuron identity and neuropsychiatric disease
PD is widely understood as a complex genetic disorder, where specific genetic risk factors predispose carriers to an increased risk of developing PD. While a handful of genes have clear mechanistic links, there is significant heterogeneity in disease. Here, Team Scherzer presents evidence for alterations in circRNA, a type of RNA transcript believed to function in regulating transcription and translation with wide-reaching effects. This work and the associated BRAINcode project represent new starting points for mechanistic follow-up and may significantly alter our understanding of key pathways relevant to PD. This and similar studies may ultimately help us understand the link for the many loci with moderate to low increased risk, and support the next steps within these pathways.
Team Accolades
- Network Spotlights: Abby Olson, Daniel El Kodsi, Xianjun Dong
- Open Science Champions: Zechuan Lin
Other Team Activities
- Working Groups:
- Single Cell Multi(Omics) – Xianjun Dong (Subgroup Lead)
- Spatial Transcriptomics – Xianjun Dong (Co-Chair)
- ANALYSE Neuropathology – Geidy Serrano (Co-Chair)
- PFF Gut-Brain – Geidy Serrano (Co-Chair)
- Interest Groups:
- Aging & Progression – Geidy Serrano (Chair)
- PD Modeling – Rodent & Fly Models – Mel Feany (Co-Chair)
In the News
- ASAP Initiative’s $9 million gift elevates Parkinson’s research (Brigham and Women’s Hospital)
- Scherzer, Feany, and Dong selected for $9M Aligning Science Across Parkinson’s initiative grant (Center for Advanced Parkinson Research, September 16, 2020)
- Brain’s hidden “junk” – mysterious RNA circles produced by cells damaged in Parkinson’s and Alzheimer’s disease (SciTechDaily, September 18, 2023)