Circuitry and Brain-Body Interactions | 2021
Mapping the Modulatory Landscape Governing Striatal Dopamine Signaling and Its Dysregulation in Parkinson’s Disease
Study Rationale: Nerve cells that produce the brain chemical dopamine die in people with Parkinson’s disease (PD). These nerve cells extend long and thin fibers called axons that release dopamine from thousands of different points, sending signals to other nerve cells in a brain area called the striatum. Many different types of cells and molecules in the striatum can directly control how dopamine is released, but researchers have not yet discovered which ones are the most important and how they are affected in Parkinson’s. By better understanding this cooperation between striatum and the release of dopamine from axons, the team could provide new knowledge toward ways to restore normal function.
Hypothesis: Team Cragg thinks that other molecules in the striatum play a very important role in controlling the release of dopamine, particularly for the types of dopamine axons that are most vulnerable in PD. The team believes that this role is disrupted in the disease and could be targeted to rescue symptoms.
Study Design: Team Cragg’s international team will combine cutting-edge research methods in mice and human cells that allow the team to study the biology behind Parkinson’s. The team will measure dopamine and other signaling molecules in different areas of the striatum and work out what they do. This work will reveal the biological differences between vulnerable and resistant areas. Team Cragg will use this knowledge to study the most promising molecules in mice that develop PD and in cells from people with PD, to then suggest new ways that might fix the problems with dopamine in the disease.
Impact on Diagnosis: Team Cragg’s discoveries will provide knowledge that may help to find new ways of treating Parkinson’s using medicines that target the key signaling molecules in striatum that control dopamine release.
Leadership
Project Outcomes
Team Cragg expects their collaboration to unravel the modulators and circuits within striatum that govern dopamine function for vulnerable versus resistant neurons, and the related dysfunction in these key circuits during disease progression, to provide fresh mechanistic rationale for new therapies for Parkinson’s disease. View Team Outcomes.
Team Outputs
Click the following icons to learn more about the team’s outputs:
Overall Contributions
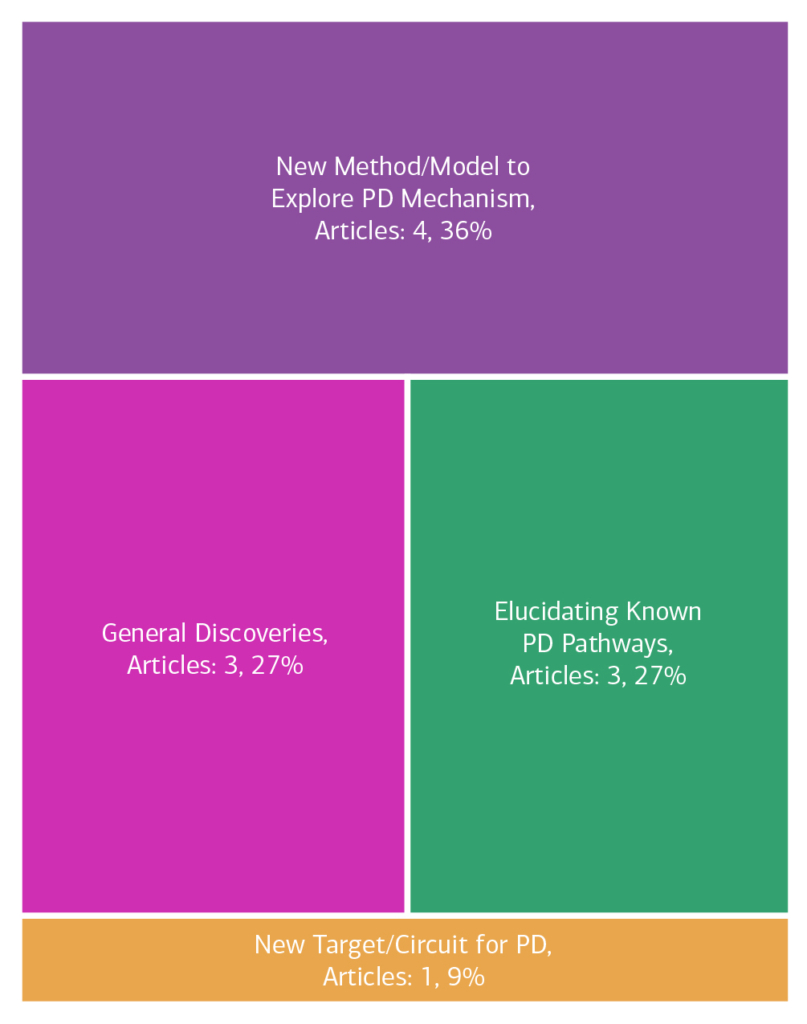
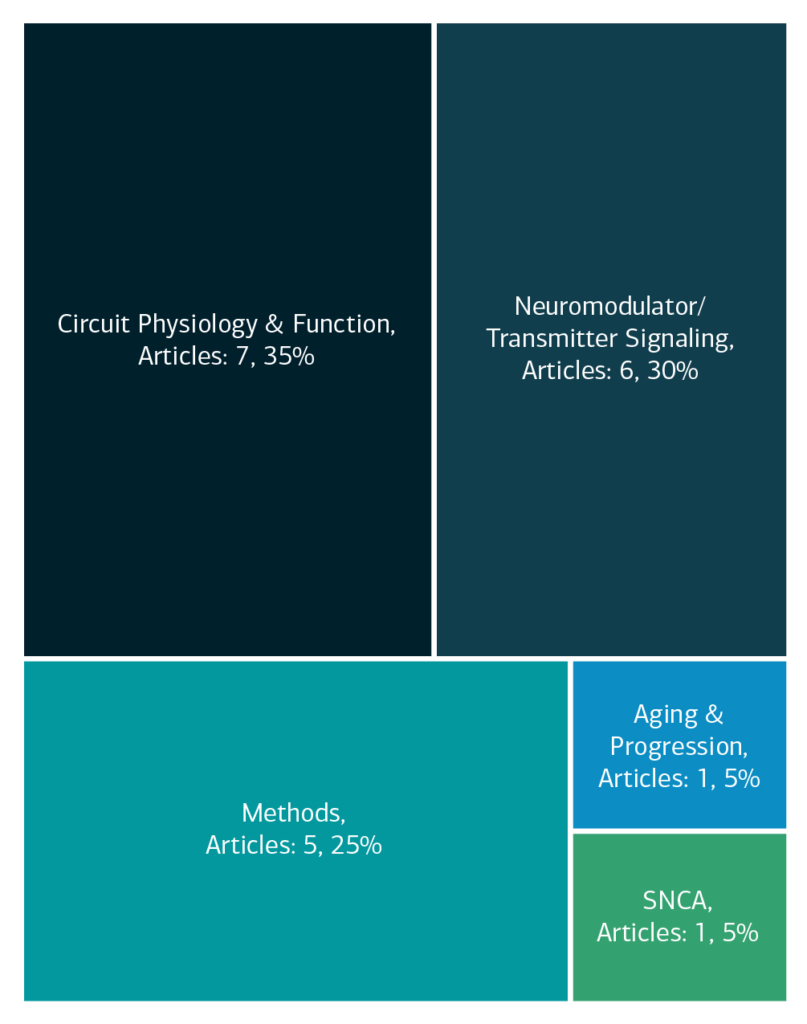
Here is an overview of how this team’s article findings have contributed to the PD field as of May 2025. There are two different categorizations of these contributions – one by impact to the PD community and a second by scientific category.
Impact
Category
Featured Output
Below is an example of a research output from the team that contributes to the ASAP mission of accelerating discoveries for PD.
Anxa1+ dopamine neuron vulnerability defines prodromal Parkinson’s disease bradykinesia and procedural motor learning impairment
This publication identifies a specific dopamine neuron subpopulation expressing Annexin A1 (Anxa1) that shows selective vulnerability in early-stage Parkinson’s disease across multiple models and in patient-derived neurons. Unlike typical dopamine neurons, Anxa1+ neurons don’t signal reward or reinforce actions, but instead correlate with vigorous movement during exploration. Silencing these neurons disrupts specific action sequences resembling PD bradykinesia symptoms. Crucially, these neurons prove essential for procedural learning and motor dexterity. The research establishes that early degeneration of Anxa1+ neurons explains characteristic prodromal PD symptoms: bradykinesia and impaired procedural motor learning. This finding advances understanding of early PD progression mechanisms.
Team Accolades
Members of the team have been recognized for their contributions.
- Open Science Champions: Peter Kilfeather, Natalie Doig, Mai-Anh Vu, Cláudia Mendes
- Network Spotlights: Cláudia Mendes
- Awards
- 2023 Collaborative Meeting: Shinil Raina (First Place in Circuitry; London), Emanuel Lopes (Second Place in Circuitry; London)
- COSA Prize Winners 2024: Ioannis Mantas (Second Place)

Other Team Activities
- Protocol Particulars Interview: Examining the Ultrastructural Components of Dopamine Neurons with Natalie Doig, PhD
- Working Groups: Spatial Transcriptomics – Dinos Meletis (Co-Chair)
- Interest Groups: Neuromodulator & Neurotransmitter Signaling – Stephanie Cragg (Chair)
In the News
- Oxford-led team given £6.6m to map uncharted networks in the progression of Parkinson’s (University of Oxford, October 27, 2021)