PD Functional Genomics | 2020
Defining the Cellular and Molecular Determinants of Variable Genetic Penetrance in Parkinson’s Disease
Study Rationale: Why do some people develop Parkinson’s disease (PD) while others do not? Although many genetic risk factors have been identified, researchers still cannot confidently answer this question, or explain how certain cells in the brain go from being healthy early in life to diseased in old age. Clearly, numerous complex factors are involved, and a systematic investigation of the key cellular and molecular players is necessary to understand and effectively treat this disease.
Hypothesis: Team Studer hypothesizes that single genetic factors are insufficient to cause PD — rather, that it is triggered by a combination of genetics, age-related factors, and their effects in different brain cells.
Study Design: Here, Team Studer proposes to dissect the genetic, age-related, and cell-type-specific factors that lead to PD using a collection of genetically diverse stem cells derived from patients. Using advanced methods pioneered by the team, they will convert these stem cells into the different types of brain cells implicated in PD — neurons, microglia, and astrocytes — allowing the team to investigate how genetic risk factors, the aging process, and these different cell types interact to trigger disease. Team Studer will assess how various combinations of these factors disrupt the function of brain cells using detailed molecular studies, microscopy, genetic manipulations, and biochemical measurements — building a computational network model of the factors that cause PD.
Impact on Diagnosis/Treatment of Parkinson’s Disease: This richer, fully human cell model of PD will provide an entirely new level of understanding of how the interplay between genetics, different brain cells, and aging shapes individual disease risk, enabling early diagnosis, prediction of therapeutic targets that could halt or reverse the disease, and stratification of patients into therapeutically meaningful subgroups.
Leadership
Project Outcomes
The project will not only help address the question of variable penetrance — why some individuals with genetic risk factors develop Parkinson’s disease while others do not — but may also lead to improved tools for early diagnosis, prediction of effective therapeutic targets, and stratification of patients into therapeutically meaningful subgroups. View Team Outcomes.
Team Outputs
Click the following icons to learn more about the team’s outputs:
Overall Contributions
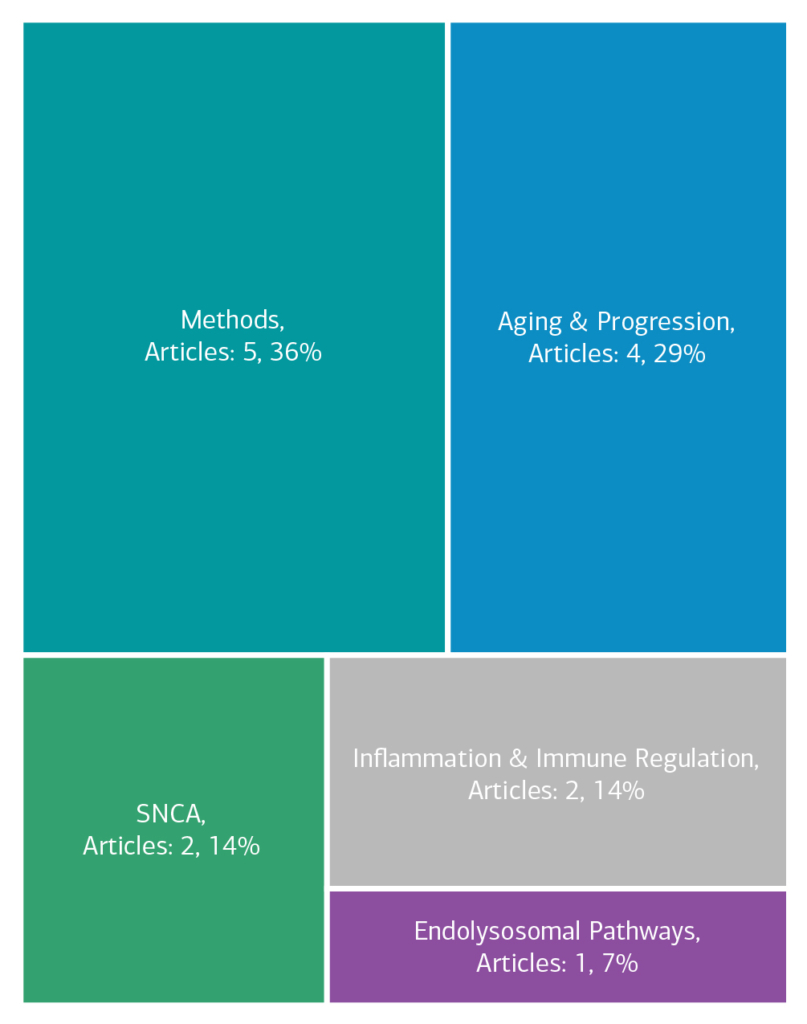
Here is an overview of how this team’s article findings have contributed to the PD field as of February 2025. There are two different categorizations of these contributions – one by impact to the PD community and a second by scientific category.
Impact

Category
Featured Output
Below is an example of a research output from the team that contributes to the ASAP mission of accelerating discoveries for PD.
Deep sequencing of proteotoxicity modifier genes uncovers a Presenilin-2/beta-amyloid-actin genetic risk module shared among alpha-synucleinopathies
Several neurodegenerative diseases are associated with misfolded proteins. However, it had yet to be determined if different diseases, characterized by misfolding of the same protein, shared genetic risk factors. Team Studer assessed the genetic sequence of patients based on their protein aggregation phenotype and identified convergent mechanisms across different synucleinopathies.
Team Accolades
Members of the team have been recognized for their contributions.
- Open Science Champions: Alex Henderson
- Awards
- COSA Prize Winners 2021: Angelique di Domenico (Reviewer Prize Winner)
Other Team Activities
- Working Groups:
- Senescence – Lorenz Studer and Isabelle de Luzy (Co-Chairs)
- iPSC – Gist Croft (Co-Chair)
In the News
- Consortium including NYSCF receives grant award to study Parkinson’s disease risk factors (The New York Stem Cell Foundation, September 18, 2020)
- Taking aim at Parkinson’s disease: A conversation with Developmental Biologist Lorenzo Studer (Memorial Sloan Kettering Cancer Center, October 9, 2020)