Circuitry and Brain-Body Interactions | 2021
Olfactory Circuits: Alpha-Synuclein-Rich Neurons Respond to Environmental Triggers at the Origin of Parkinson’s Disease
Study Rationale: To slow the progression of Parkinson’s disease (PD), researchers need to know more about how it starts. It is well known now that many individuals with PD have had a significant reduction in their ability to smell years before their movement disorder starts. Here, Team Schlossmacher will study the role of a PD-linked gene, alpha-synuclein, in odor processing, and how viruses in the nose may change how alpha-synuclein is handled within the odor-signaling cells of the brain in people with PD.
Hypothesis: Team Schlossmacher hypothesizes that certain environmental triggers, including viruses, can start a chain reaction inside the nasal cavity by which a protein called alpha-synuclein begins to form insoluble clumps. This happens inside the nerve circuits that specifically process scent and then gradually spreads further inside the brain, thereby promoting Parkinson’s disease.
Study Design: Team Schlossmacher will first define the normal role of the alpha-synuclein protein in the scent-processing circuits of mice and humans. The team will then answer whether so called Lewy bodies (i.e., alpha-synuclein clumps in nerve cells) contribute to the inability to smell. Third, they will study the areas of the human brain that are responsible for smell, including using MRI imaging. Lastly, Team Schlossmacher will perform studies with nasal fluids from people with PD and introduce these (and viruses) into mice, to see if these start a reaction that cause changes in the mouse olfactory system like what is seen in humans with PD, including loss of smell and changes in the alpha-synuclein protein. The team will see if the inability to smell can progress to motor deficits.
Impact on Diagnosis: This study will delineate what causes the loss of sense of smell in PD. That will help researchers to develop new drugs to treat it and explore new ways to diagnose the disease, hopefully at a stage before the movement symptoms appear. Team Schlossmacher may also gain insights into risk factors for PD, which could lead to strategies to help people reduce their risk. The team will also create new animal models that can be used by others.
Leadership
Project Outcomes
Team Schlossmacher will determine whether environment-a-synuclein interactions and inflammation in the nasal cavity can act as triggers for disease initiation, for a-synuclein aggregate formation and associated neuronal circuit dysfunction, thus informing risk and progression of disease and biomarker development in humans. View Team Outcomes.
Team Outputs
Click the following icons to learn more about the team’s outputs:
Overall Contributions
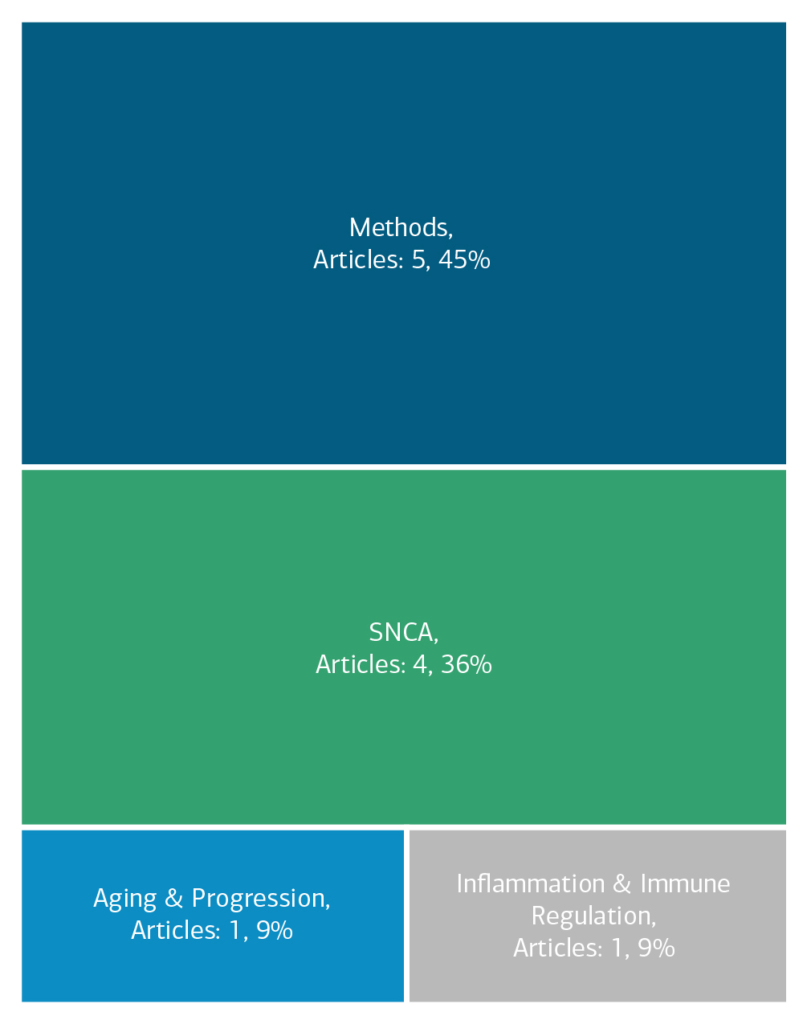
Here is an overview of how this team’s article findings have contributed to the PD field as of November 2023. There are two different categorizations of these contributions – one by impact to the PD community and a second by scientific theme.
Impact

Theme
Featured Output
Below is an example of a research output from the team that contributes to the ASAP mission of accelerating discoveries for PD.
Constitutive nuclear accumulation of endogenous alpha-synuclein in mice causes motor impairment and cortical dysfunction, independent of protein aggregation
The team engineered Snca-NLS mice which localize endogenous aSyn to the nucleus. Characterization of Snca-NLS mice indicates that an increase of nuclear aSyn is sufficient to elicit PD-like phenotypes, including age-dependent motor impairment and cortical dysfunction. The results suggest that chronic endogenous nuclear aSyn can elicit toxic phenotypes in mice, independent of its aggregation. This model raises key questions related to the mechanism of aSyn toxicity in PD and provides a new system to study an under-appreciated aspect of PD pathogenesis.
Team Accolades
Members of the team have been recognized for their contributions.
- Open Science Champions: Maxime Rousseaux
Other Team Activities
- Updates will be posted when available.
In the News
- Does Parkinson’s start in the nose? International team awarded US$9 million ASAP grant to find out (University of Ottawa, press release, October 26, 2021)
- Aligning Science Across Parkinson’s initiative awards $132 million (Philanthropy News Digest, October 28, 2021)