PD Functional Genomics | 2020
IMPACT-PD – Implications of Polyamine and Glucosylceramide Transport in Parkinson’s Disease
Study Rationale: Mutations in the genes ATP13A2 (PARK9) and ATP10B trigger Parkinson’s disease (PD) and cause dysfunction of lysosomes, the recycling compartments of the cell. Team Vangheluwe explained these defects by impaired transport of polyamines and glucosylceramide out of the lysosome, respectively. Polyamines are cell protective agents, whereas the levels of the lipid glucosylceramide are controlled by GBA1, the major genetic risk factor of PD. However, there is a clear knowledge gap regarding the biology of polyamine and glucosylceramide transport systems in neurons and their supporting cells of the brain, and how an impaired polyamine and glucosylceramide distribution in these cells leads to neurodegeneration.
Hypothesis: Team Vangheluwe hypothesizes that an impaired polyamine and glucosylceramide transport activity causes toxic accumulation of these substances in lysosomes and leads to a shortage elsewhere in the cell. Together, this may cause lysosomal and mitochondrial dysfunction, and lead to α-synuclein toxicity, three major hallmarks of PD.
Study Design: First, Team Vangheluwe will investigate the molecular architecture of polyamine and glucosylceramide transporters and identify mechanisms to modulate their activity. Second, the team will examine how these transporters influence the intracellular distribution of polyamine and glucosylceramide, and how this affects the cross-talk between lysosomes and mitochondria. Third, they will investigate how dysfunctional polyamine and glucosylceramide transporters affect other PD pathways, such as mitophagy, GBA1 and alpha-synuclein aggregation, and whether the modulation of these transporters can be validated as therapeutic approach for PD. Finally, Team Vangheluwe will collect evidence for disturbed polyamine and glucosylceramide transport in PD patients.
Impact on Diagnosis/Treatment of Parkinson’s Disease: Team Vangheluwe will validate the neuroprotective effect of polyamine and glucosylceramide transporters and investigate their potential to reverse α-synuclein and GBA1 pathology. This may offer new therapeutic strategies that correct aberrant lysosomal and mitochondrial dysfunction in Parkinson’s disease. The team will analyze whether alterations in the polyamine and glucosylceramide levels together may be considered as biomarkers for PD.
Leadership
Project Outcomes
By dissecting the neuroprotective effect of lysosomal polyamine and glucosylceramide transporters at the molecular level, Team Vangheluwe will establish new pathways implicated in Parkinson’s disease that may serve as novel therapeutic targets to restore lysosomal dysfunction in Parkinson’s disease. View Team Outcomes.
Team Outputs
Click the following icons to learn more about the team’s outputs:
Overall Contributions
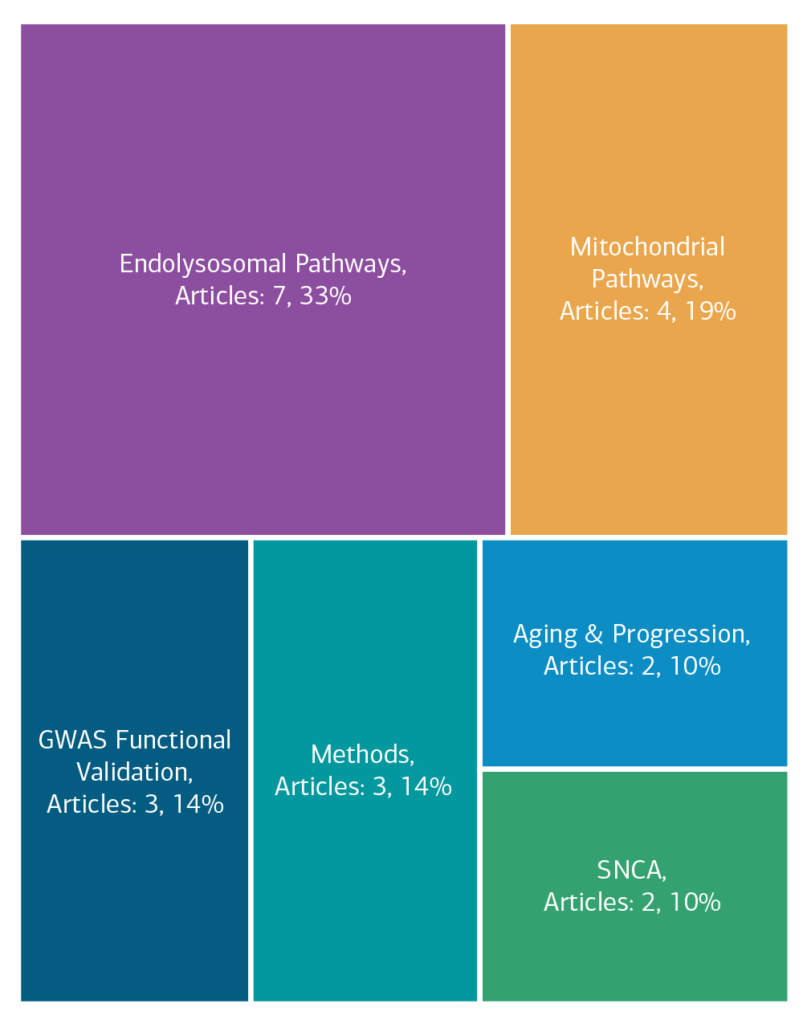
Here is an overview of how this team’s article findings have contributed to the PD field as of May 2025. There are two different categorizations of these contributions – one by impact to the PD community and a second by scientific category.
Impact

Category
Featured Output
Below is an example of a research output from the team that contributes to the ASAP mission of accelerating discoveries for PD.
Cholesterol-mediated Lysosomal Dysfunction in APOE4 Astrocytes Promotes α-Synuclein Pathology in Human Brain Tissue
A hallmark feature of neurodegenerative diseases, such as Parkinson’s disease, is an aggregation of proteins in cells and subsequent formation of inclusions. To enhance our understanding of the mechanisms behind this aggregation, it is necessary to utilize model systems that reliably recapitulate context found in the human brain. Team Vangheluwe expanded upon a recent iPSC-derived human 3D brain tissue known as miBrain, which incorporates major cell types found in the brain in a way that is anatomically precise and capable of modeling neurodegeneration. The updates that the team incorporated improved scalability and reduced variability. Through the use of this system, the team was able to investigate the relationship between AO4 and alpha-synuclein phosphorylation and aggregation in human brain tissue, discovering that APOE4 increases alpha-synuclein phosphorylation through lipid accumulation in astrocytes.
Team Accolades
Members of the team have been recognized for their contributions.
- Open Science Champions: Marine Houdou
- Awards
- COSA Prize Winners 2021: Stephanie Vrijsen (Community Prize Winner)
- COSA Prize Winners 2022: Eduard Bentea (Second Place)
- 2023 Collaborative Meeting: Eduard Bentea (Third Place Winner in Functional Genomics; San Diego)

Other Team Activities
In the News
- Check back soon for updates from Team Vangheluwe.